On November 2, 2021 the Centers for Medicare and Medicaid Services (CMS) released the 2022 Ambulatory Surgical Center Quality Reporting Program (ASCQR) Final Rule. The 1,394 page final rule contains many changes that will take place in the 2022 ASCQR performance year and beyond.
This blog post breaks down the finalized changes to the ASCQR.
2022 Final Changes
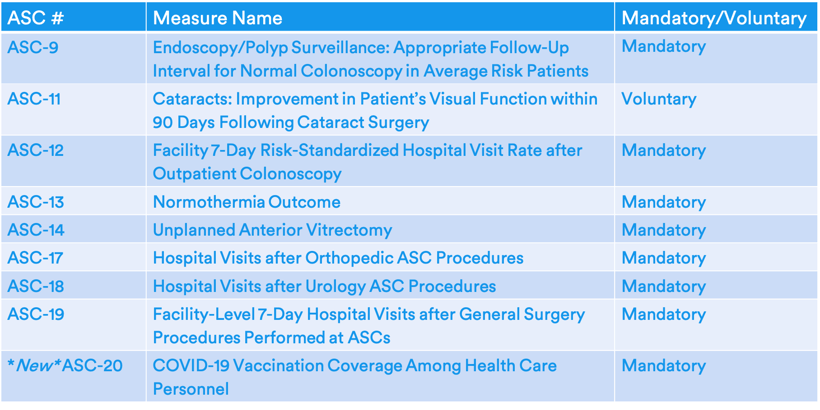
For the 2022 reporting year/2024 payment year, there will be a new measure: ASC-20: COVID-19 Vaccination Among Health Care Personnel (HCP). ASC-20 will measure the percent of HCP eligible to work in the ASC for at least one day during the reporting period who received a complete COVID-19 vaccination course. The measure specification lists an exception for those with contraindications to COVID-19 vaccination that are described by the CDC.
As finalized, for the 2022 reporting period, measure data would need to be collected, submitted, and scored as follows:
- Collect: ASCs will need to collect numerator and denominator data this measure for at least one self-selected week during each month of the reporting quarter.
- Submit: Before the end of each quarter, ASCs will need to submit this data to the NHSN Healthcare Personal Safety (HPS) Component.
- Score: The CDC will then calculate a single quarterly COVID-19 HCP vaccination rate for each ASC using the average of the ASC's submitted data from that quarter.
- Public Reporting on Care Compare: CMS will then publish the rate of only the most recent quarter so that only the most up-to-date vaccination rates are available.
Analysis: Although this measure will, to some extent, increase ASCQR reporting burden, most ASCs should be familiar with the NHSN system from the old ASCQR Influenza Immunization measure. Given the close contact between HCP and patients in ASCs, risk of COVID-19 transmission is high for both HCPs and patients. CMS is finalizing this measure to ensure that ASCs are "taking steps to limit the spread of COVID-19".
Final 2022 ASCQR Measure Set
2023 Final Changes
CMS finalized significant changes to the ASCQR requirements for the 2023 reporting period/2025 payment year.
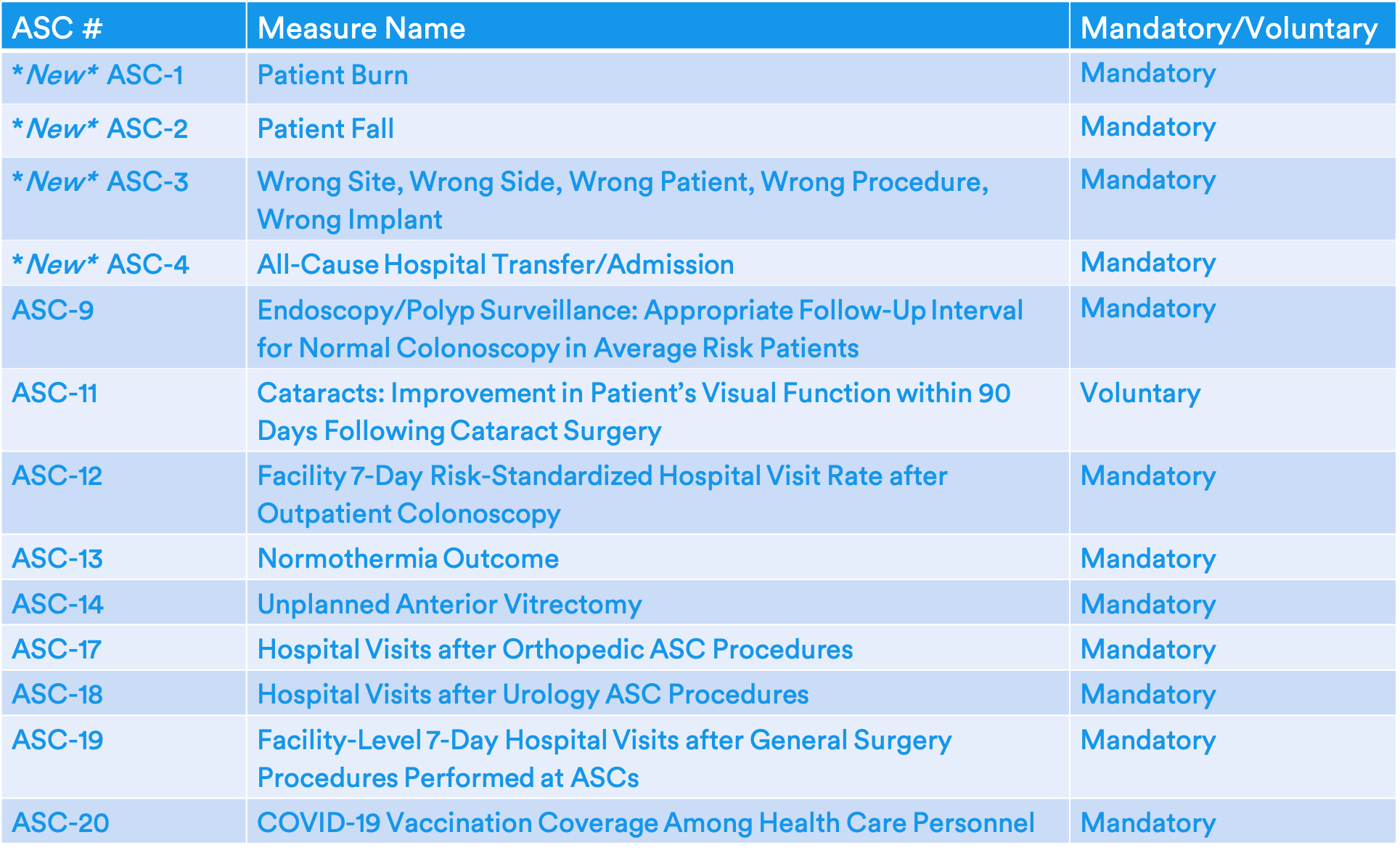
- Measures ASC-1, 2, 3, and 4 will be required once again.
- Data collection on these measures was suspended in 2019 rule because the measures were topped out and because the data submission method, which involved adding specific QDCs onto eligible claims, was impacting the completeness and accuracy of the data.
- For 2023, CMS will require providers to submit measure data via the HQR System (formerly referred to as the QualityNet Secure Portal), rather than via claims. Because of this, these measures will now be required to be reported on all patients, not just Medicare patients.
Final 2023 ASCQR Measure Set
2024 Final Changes
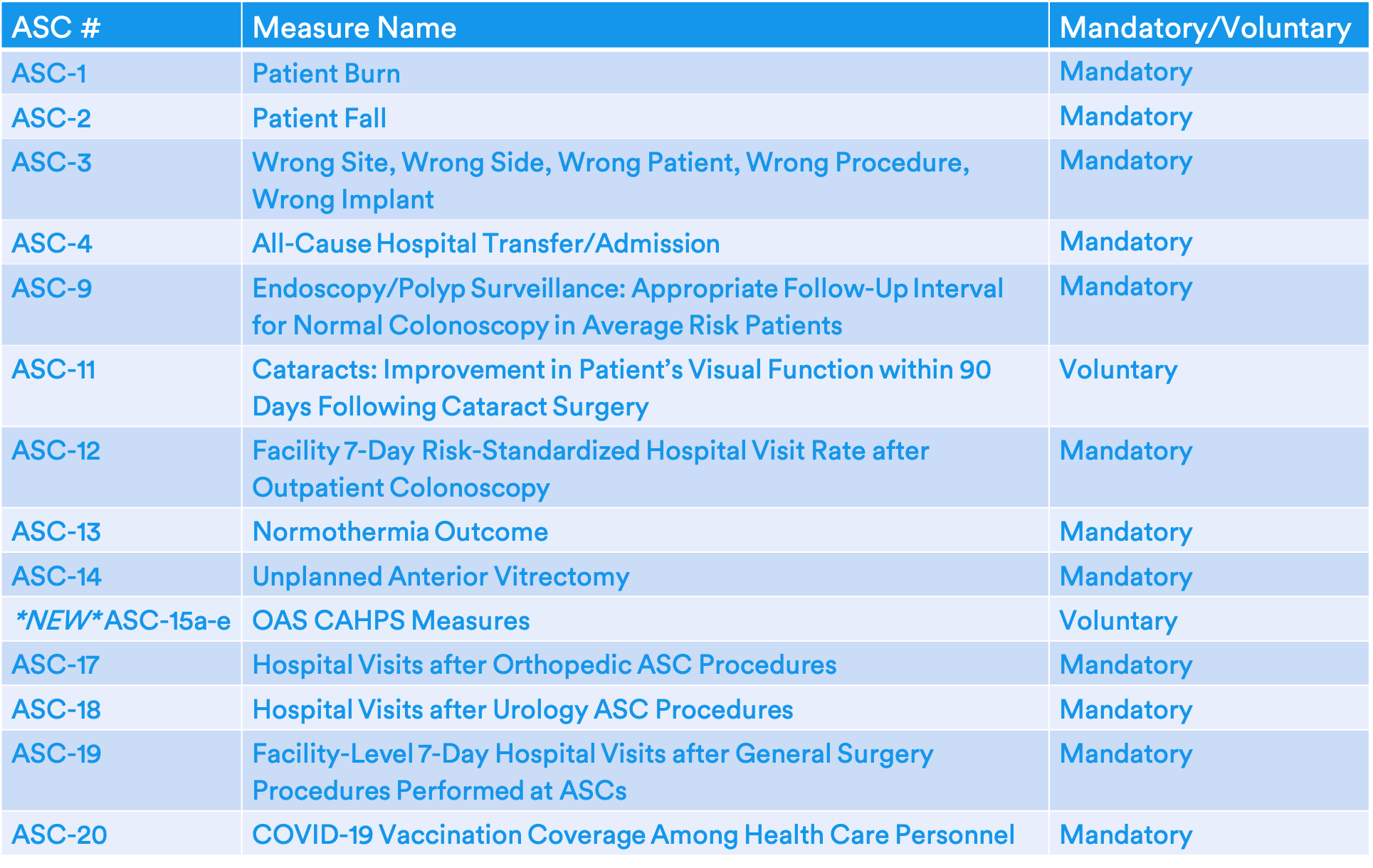
CMS is finalizing their proposal to allow voluntary reporting of ASC-15a-e: Outpatient Ambulatory Surgery (OAS) CAHPS Survey measure. The original proposal was to implement voluntary reporting in 2023 but, in response to staffing and burden concerns due to the pandemic, CMS has finalized a one-year delay.
- CMS has finalized additional collection modalities using a web-based module (web with mail follow-up of non-respondents and web with telephone follow-up of non-respondents) for administering the survey. CMS believes this will address concerns about burden and cost of survey administration.
Final 2024 ASCQR Measure Set
2025 Final Changes
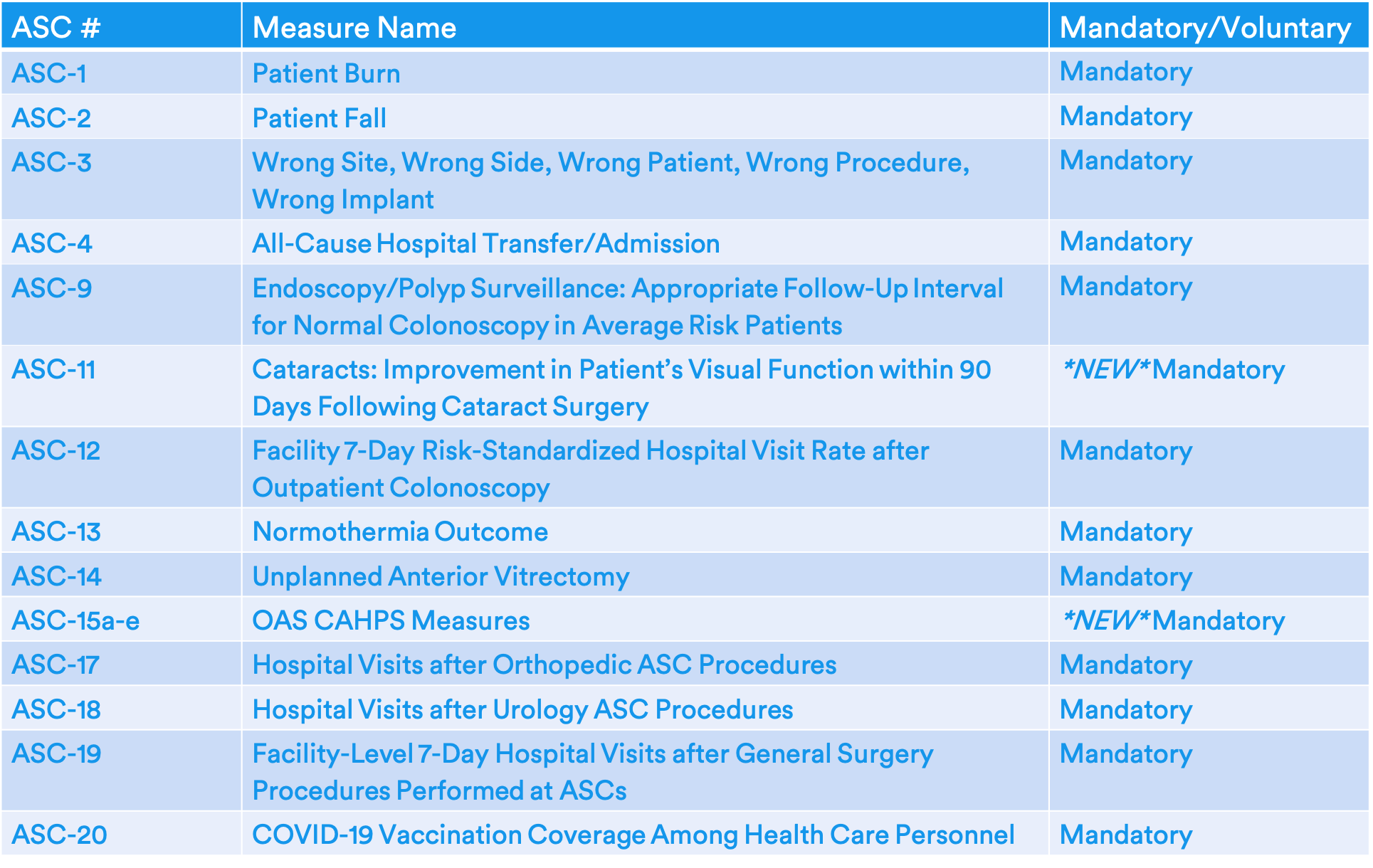
CMS finalized mandatory reporting on two previously voluntary measures -- ASC-11 and ASC-15a-e.
ASC-11: Cataracts: Improvement in Patient's Visual Function within 90 Days Following Cataract Surgery
- In response to a significant number of stakeholder concerns, CMS delayed the original proposal (which would have required reporting in 2023) by two years to the 2025 reporting year.
- This measure has been voluntary for years.
- Ophthalmic specialty societies have opposed this due to the inappropriate nature of these surveys being attributed to the ASC facility rather than to the individual surgeon and the burdensome nature of patient surveys, particularly in this context. This would require the ASC to report on data that is located in the surgeon's office and, thus, inaccessible by the ASC as, per Medicare ASC Conditions for Coverage, the two entities must be physically, administratively, and financially separate from one another.
- We anticipate this measure will continue to be challenged in future years.
ASC-15a-e: Outpatient Ambulatory Surgery (OAS) CAHPS Survey measure
- In response to significant concerns about burden, CMS delayed the original proposal (which would have required reporting in 2024) by one year to the 2025 reporting year.
- This measure is burdensome and many ASCs struggle to convince patients to complete any surveys, let alone lengthy 37 to 52 question surveys.
- We anticipate there will continue to be significant opposition to mandating this measure in its current form.
Final 2025 ASCQR Measure Set
Next Steps
Share this information with your colleagues. If you want hands-on, personalized assistance, contact us and we will have your back.

